NCC presenting the success story
NCC Spain
💡Success story about simulating cardiac functions💡
📋 "Atrial Electromechanical Modeling for In Silico Assessment of Atrial Fibrillation Therapies" developed by Sergi Picó Cabiró, Alberto Zingaro, Eva Casoni and Mariano Vazquez from ELEM Biotech, in collaboration with Violeta Puche García, Francisco Javier Saiz Rodriguez and Lucía Romero Pérez from Ci2B - Center for Research and Innovation in Bioengineering
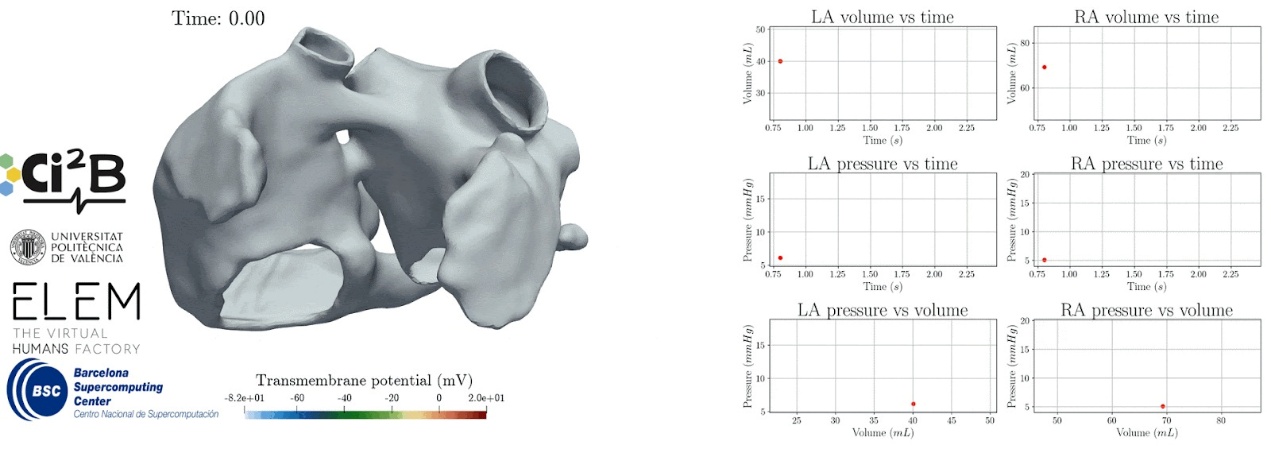
Heart's atria are the two upper chambers of the heart and play a central role in cardiac function. They are also involved in cardiac conditions like atrial fibrillation, so a high fidelity fidelity computational model that captures the complex electromechanical behavior of the human atria is key to understand and treat these disorders.
The team developed a model combining detailed electrophysiological dynamics with mechanical features of atrial tissue, allowing to simulate the full cycle of atrial function: reservoir, conduit, and booster phases. It accounts for both anatomical and electrical heterogeneity and it is coupled to a simplified circulatory model to reflect pressure and flow dynamics across the heart.
🖥️ Thanks to RES supercomputer hashtag#MareNostrum5 from Barcelona Supercomputing Center, they used the Alya multiphysics simulation code to carry out large-scale simulations with high spatial and temporal resolution. The model has been calibrated and validated against literature clinical data, including key physiological biomarkers like atrial volumes and ejection fractions.
One of the main goals is to use this modeling framework to explore treatment strategies in silico for atrial fibrillation, such as antiarrhythmic drugs and catheter ablation. By simulating how individual patients might respond to specific interventions, this approach moves us closer to truly personalized treatment planning in cardiology.
CLIENT/USER PROFILE:
Researchers and scientists in the field of cardiology, biomedical engineering, and computational modeling, particularly those involved in the study of atrial fibrillation and the development of new treatments.
IMPACT:
The project has the potential to significantly impact the understanding and treatment of atrial fibrillation by providing a high-fidelity computational model that captures the complex electromechanical behavior of the human atria.
BENEFITS:
Advancements in cardiac modeling: The project demonstrates the development of a comprehensive computational model that simulates the full cycle of atrial function, including reservoir, conduit, and booster phases.
Personalized treatment planning: The model has the potential to be used for in silico assessment of treatment strategies for atrial fibrillation, allowing for personalized treatment planning and potentially improving patient outcomes.
Improved understanding of atrial fibrillation: The project provides valuable insights into the complex electromechanical behavior of the human atria, shedding light on the underlying mechanisms of atrial fibrillation.
KEY POINTS BEFORE AGREEING ON THE PROJECT:
Clear objectives: Defining the project's goals, including the specific research questions to be addressed and the expected outcomes.
Computational resources: Ensuring access to sufficient computational resources, such as the MareNostrum5 supercomputer, to perform the required simulations.
Interdisciplinary collaboration: Collaborating with experts from various fields, including cardiology, biomedical engineering, and computational modeling, to ensure the project's success.
TECHNICAL/SCIENTIFIC CHALLENGE:
The project faced the challenge of developing a comprehensive computational model that captures the complex electromechanical behavior of the human atria, including anatomical and electrical heterogeneity.
SOLUTION:
The team utilized the Alya multiphysics simulation code on the MareNostrum5 supercomputer to develop a model that combines detailed electrophysiological dynamics with mechanical features of atrial tissue. The model was calibrated and validated against literature clinical data, including key physiological biomarkers like atrial volumes and ejection fractions. The results demonstrate the potential of this modeling framework for in silico assessment of treatment strategies for atrial fibrillation.
📷 The image shows a simulation of the electromechanical activity of the atria and pv loops
This project is a collaboration between Ci2B - Center for Research and Innovation in Bioengineering, Barcelona Supercomputing Center and ELEM Biotech