NCC presenting the success story
NCC Spain
💧💡A RES Success Story aimed to improve the water stability of amine-appended MOFs💡💧
📋 “Enhancing the water stability of Titanium Organic Frameworks with grafted amines for CO2 Capture” by Carlos Martí-Gastaldo's from Instituto de Ciencia Molecular- ICMol/Universitat de València
Directly capturing CO₂ from air is rapidly advancing, particularly developing new amine-appended metal-organic frameworks (MOFs). Previous studies revealed an alternative grafting mode of amines in the MUV-10 material and clarified the underlying mechanism. However, the stability of the final material under air and up to 60% humidity depends on the amine size.
🖥️ Thanks to RES supercomputer #MareNostrum5 from Barcelona Supercomputing Center, the team conducted simulations to investigate the experimental differences in the stability of MUV-10–amine materials. The results helped to guide the design of new amine-appended MOFs with improved stability and increased CO₂ affinity.
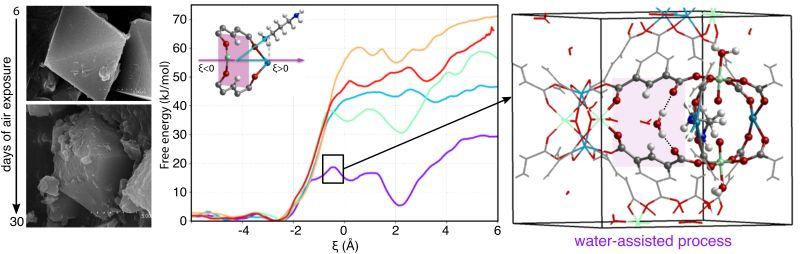
The diffusion of amines through the cages and cavities of MUV-10 involves the temporary breaking of two Ca–O bonds to widen the crossing ring. After the amine has passed through, these bonds are restored. The team's umbrella sampling simulations suggest that water molecules significantly stabilize the transition state of the process by forming hydrogen bonds with both the oxygen atoms of the linker and the amine groups of the alkylamine.
For large amines, the combination of high adsorption energies and increased diffusion barriers helps to trap them effectively. This controlled diffusion allows the passing ring to properly reconfigure without disrupting the material’s crystallinity. In contrast, increasing the diffusion of small-chain amines shows that water may play a crucial role in triggering an irreversible structural collapse.
CLIENT/USER PROFILE:
Researchers and scientists in the field of materials science, chemistry, and environmental engineering, particularly those involved in the development of new materials for CO2 capture and storage.
IMPACT:
The project has the potential to significantly impact the development of new materials for CO2 capture and storage, particularly in the context of climate change mitigation.
BENEFITS:
Improved understanding of MOF stability: The project provides valuable insights into the factors that affect the stability of amine-appended MOFs, including the role of water and amine size.
Design of new MOFs with improved stability: The results of the project can inform the design of new MOFs with improved stability and CO2 affinity, which can be used for CO2 capture and storage.
Advancements in CO2 capture technology: The project contributes to the development of new technologies for CO2 capture and storage, which are critical for mitigating climate change.
KEY POINTS BEFORE AGREEING ON THE PROJECT:
Clear objectives: Defining the project's goals, including the specific research questions to be addressed and the expected outcomes.
Computational resources: Ensuring access to sufficient computational resources, such as the MareNostrum5 supercomputer, to perform the required simulations.
Interdisciplinary collaboration: Collaborating with experts from various fields, including materials science, chemistry, and environmental engineering, to ensure the project's success.
TECHNICAL/SCIENTIFIC CHALLENGE:
The project faced the challenge of understanding the factors that affect the stability of amine-appended MOFs, particularly in the presence of water.
SOLUTION:
The team utilized simulations on the MareNostrum5 supercomputer to investigate the experimental differences in the stability of MUV-10–amine materials. The results showed that water molecules play a crucial role in stabilizing the transition state of the amine diffusion process, and that the combination of high adsorption energies and increased diffusion barriers helps to trap large amines effectively. The study provides valuable insights into the design of new MOFs with improved stability and CO2 affinity.
📸 The image shows MUV-10-DAP crystals (left) viewed with scanning electron microscopy after 6 and 30 days of air exposure. Free energy profiles for the diffusion of the amine with different conditions (middle), snapshot of the transition state stabilized by the presence of the water (right).