NCC presenting the success story
NCC Spain
💡Success Story about new reactivity predictions💡
📋 "Exploring the enantioconvergent polycyclization of linear terpenoids catalyzed by Squalene Hopene Cyclase" by the group of Sílvia Osuna from Universitat de Girona
Enzymes can be used to uniquely perform reactions in a selective manner. Recently, in collaboration with Prof. Hauer's team from Johannes Gutenberg-Universität Mainz, they reported the ability of aaSHC (a membrane protein from *Alicyclobacillus acidocaldarius*) to, starting from a 50/50 mixture, produce the exact same single stereoproduct of the industrially-relevant compound Ambroxide.
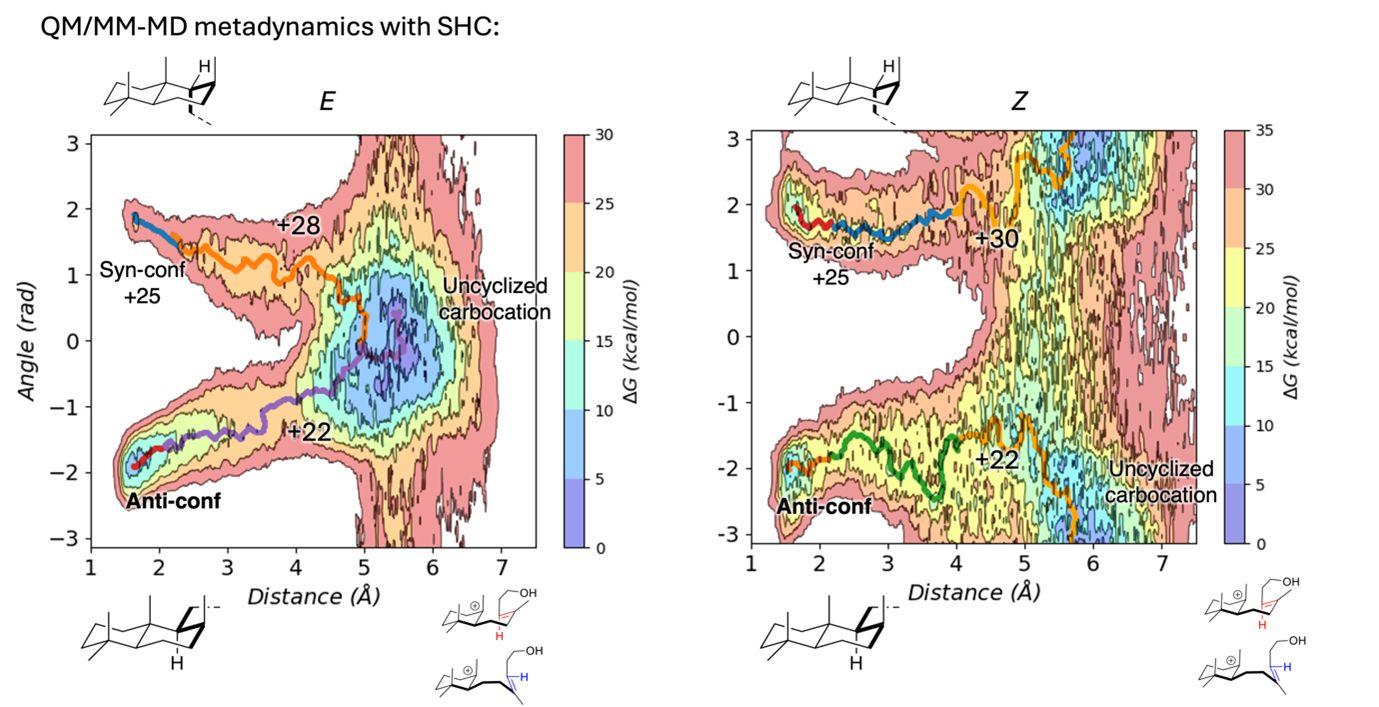
The project focused on exploring the cyclization of both homofarnesol isomers using QM/MM metadynamics simulations, given the potential that this amazingly rare phenomenon held.
🖥️ Thanks to RES supercomputer #MareNostrum5 from Barcelona Supercomputing Center, the team could calculate more than 6 nanoseconds of metadynamics, producing a detailed free energy surface of the process.
The free energy barriers confirmed that the process is enantioconvergent. In comparison with the equivalent gas-phase reaction, the active site pocket of the protein restricts the flexibility of homofarnesol and favors the production of only one of the stereoproducts. They will further study which specific amino acids of the enzyme are responsible for this phenomenon.
CLIENT/USER PROFILE:
Researchers and scientists in the field of enzymology, biocatalysis, and computational chemistry, particularly those involved in the study of enzyme-catalyzed reactions and the development of new biocatalytic processes.
IMPACT:
The project has the potential to significantly impact the field of biocatalysis and the production of industrially relevant compounds, such as Ambroxide, by enabling the development of more efficient and selective enzymatic processes.
BENEFITS:
Understanding enzyme-catalyzed reactions: The project provides valuable insights into the mechanisms of enzyme-catalyzed reactions, particularly the enantioconvergent polycyclization of linear terpenoids.
Development of new biocatalytic processes: The results of the project can inform the development of new biocatalytic processes with improved selectivity and efficiency.
Advancements in computational chemistry: The project demonstrates the power of computational chemistry tools, such as QM/MM metadynamics simulations, in understanding complex biochemical processes.
KEY POINTS BEFORE AGREEING ON THE PROJECT:
Clear objectives: Defining the project's goals, including the specific research questions to be addressed and the expected outcomes.
Computational resources: Ensuring access to sufficient computational resources, such as the MareNostrum5 supercomputer, to perform the required simulations.
Interdisciplinary collaboration: Collaborating with experts from various fields, including enzymology, biocatalysis, and computational chemistry, to ensure the project's success.
Technical/Scientific Challenge:
The project faced the challenge of understanding the mechanisms of enantioconvergent polycyclization of linear terpenoids catalyzed by Squalene Hopene Cyclase, a complex biochemical process.
SOLUTION:
The team utilized QM/MM metadynamics simulations on the MareNostrum5 supercomputer to study the cyclization of homofarnesol isomers, producing a detailed free energy surface of the process. The simulations revealed that the active site pocket of the protein restricts the flexibility of homofarnesol and favors the production of only one of the stereoproducts, confirming the enantioconvergent nature of the process.
📸 The image shows the free energy landscapes of the polycyclization of homofarnesol, either from the cis or trans configuration, in the active site of Squalene Hopene Cyclase (AaSHC). All energies are expressed in kcal/mol.