NCC presenting the success story
NCC Spain
💡 Success Story about Advancing Neurodegenerative Disease Research💡🧠
📋 "LRP1's extended glycosylation may affect the protein's quaternary structure formation and stability" led by Giuseppe Battaglia from Institute for Bioengineering of Catalonia (IBEC)
The brain is the most energy-consuming organ in the human body, needing a unique, specialized vasculature. That isolates the brain from the rest of the organism, the so-called 'blood-brain barrier' (BBB), controlling the passage of molecules to and from the brain.
Receptor LRP1 is a glycoprotein consisting of 4544 aminoacids which holds special interest as its malfunctioning is involved in many neurodegenerative diseases, such as Alzheimer's. However, LRP1's crystal structure is still unknown and limits potential therapeutic treatments.
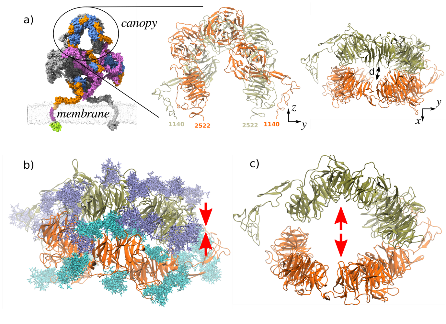
The structure of membrane protein LRP2, which is closely related lo LRP1, has been resolved and is used as a template for proposing new LRP1 models using homology modelling. This model suggests that LRP1 can adopt a coiled conformation and form homodimers (a union of two identical molecules) in the canopy, a highly sugar-dense region. This protein modification adds long, branching sugar chains which are unique in humans compared to other organisms.
🖥️ Thanks to RES supercomputer #Cibeles from Universidad Autónoma de Madrid, the team performed atomistic Molecular Dynamics to analyze the canopy region of LRP1.
Simulations revealed glycans act as “dimeric glue,” stabilizing LRP1's canopy by reinforcing protein-protein interactions. Without them, the structure destabilizes, which suggests that glycans play an active role in maintaining LRP1's quaternary structure and, in consequence, in neurodegenerative disease mechanisms.
CLIENT/USER PROFILE:
Researchers and scientists in the field of neurodegenerative diseases, structural biology, and biochemistry, particularly those involved in the study of LRP1 and its role in Alzheimer's disease.
IMPACT:
The project has the potential to significantly impact our understanding of LRP1's structure and function, and its role in neurodegenerative diseases such as Alzheimer's.
BENEFITS:
Advancements in LRP1 research: The project provides valuable insights into the structure and function of LRP1, including the role of glycosylation in its quaternary structure formation and stability.
Understanding the mechanisms of neurodegenerative diseases: The results of the project shed light on the potential mechanisms by which LRP1 malfunctioning contributes to neurodegenerative diseases.
Potential therapeutic targets: The study identifies potential therapeutic targets for the treatment of neurodegenerative diseases, such as modulating LRP1's glycosylation.
KEY POINTS BEFORE AGREEING ON THE PROJECT:
Clear objectives: Defining the project's goals, including the specific research questions to be addressed and the expected outcomes.
Computational resources: Ensuring access to sufficient computational resources, such as the Cibeles supercomputer, to perform the required simulations.
Interdisciplinary collaboration: Collaborating with experts from various fields, including structural biology, biochemistry, and neuroscience, to ensure the project's success.
TECHNICAL/SCIENTIFIC CHALLENGE:
The project faced the challenge of understanding the complex structure and function of LRP1, including the role of glycosylation in its quaternary structure formation and stability.
SOLUTION:
The team utilized atomistic Molecular Dynamics simulations on the Cibeles supercomputer to analyze the canopy region of LRP1. The simulations revealed that glycans act as "dimeric glue," stabilizing LRP1's canopy by reinforcing protein-protein interactions. The results suggest that glycans play an active role in maintaining LRP1's quaternary structure and, in consequence, in neurodegenerative disease mechanisms.
📷 The image shows:
🔹 The canopy region (Fig. 1.a), where dense glycosylation stabilizes LRP1 dimerization.
🔹 Glycan-protein interactions preserve structure (Fig. 1.b), while their absence disrupts protein contacts, causing conformational changes and destabilization (Fig. 1.c). This highlights glycans’ crucial role in maintaining quaternary structure.